
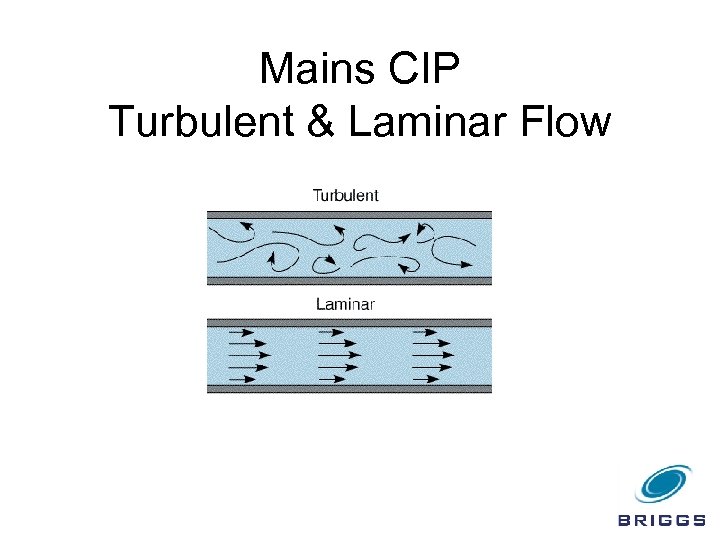
The kinetic energy equates to whether the required flow of solution in the pipework is laminar or turbulent, or somewhere in between. The effect of temperature optimisation on fats, sugars and salts is good, while on proteins it is fair.

In general, the higher the temperature the more effective the cleaning. As a rule, the variables to focus on are: temperature, time, the flow rates, method of application and the chemical solution or detergent used (see table 1).
Cip sip definition manual#
It should not be confused with sterilise in place (SIP), which is used to sanitise, disinfect and sterilise equipment, sometimes after CIP, to remove any remaining microbiological contamination wash in place (WIP), which is usually regarded as requiring more manual intervention and has less stringent validation requirements and clean out of place (COP), which entails removal of equipment from its area of operation for cleaning.Īdvantages of CIP include a reduction of cleaning time automatic cycles to ensure every item is cleaned every time increased productivity through reduction of downtime reduced chemical handling and simplified operational parameters.Įach processing installation is a custom-built unit, so the CIP regime it requires varies from application to application. Importantly, it has repeatable, reproducible and controllable results that can be validated. It relies on the principal of applying a suitable detergent or solvent at a suitable flow and/or kinetic energy application, pressure and temperature for a determined amount of time to ensure effective cleaning of the system. But too often it is not a major consideration in the initial process design.Ĭlean in place (CIP) is a method of cleaning equipment with minimal dismantling and operator/manual involvement.

A typical re-use system pharmaceutical CIP unitĮffective cleaning of process lines and equipment is critical to good manufacturing performance, reducing downtime and assured batch quality.


 0 kommentar(er)
0 kommentar(er)
